Fin regeneration in Zebrafish
Zebrafish can regenerate many of their organs, including the fins. After amputation, the fins re-grow to approximately the previous size and structure, with some plasticity in the ray bifurcation pattern. How this regenerative growth is controlled and influenced by hydrodynamic forces while swimming we study in this project together with the Jazwinska lab at the University of Fribourg.

Time-lapse imaging of the same fin during the regeneration process. The uncut original fin, prior to amputation. At 1 day post-amputation (dpa), white tissue above the amputation consists of the wound epidermis and a few blastema cells. At 3 dpa, a white excrescence above the amputation plane contains the blastema, which, despite its uniform appearance, exhibits subdivisions at the cellular and molecular level. At 6 dpa, the outgrowth extends very rapidly; the white apical tissue is maintained at the fin margin as a growth zone, while the proximal part of the outgrowth starts to display bone structures and pigmentation, which are the macroscopic markers of tissue re-differentiation. At 12 dpa, fin regeneration is at its advanced stage. At 20 dpa, the size of the fin nearly reached its original size and pattern.
This regeneration shows remarkable plasticity in the presence of altered hydrodynamic forces, both in overall shape of the fin as well as in the positioning of ray bifurcation points.
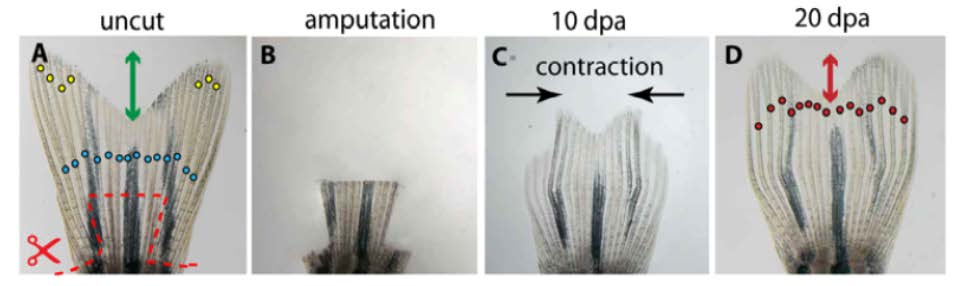
(A) Uncut fin before amputation. Plane of amputation (dashed line). Primary bifurcations (blue circles) and the depth of the middle cleft relative to the distal end of the lateral lobes (blue arrow).
(B) The stump of the fin shortly after amputation and ablation of three lateral rays.
(C) At 10 dpa, the unevenly growing regenerate with retarded lateral rays spontaneously contracts towards the midline.
(D) At 20 day, the size of the fin is fully restored. The middle cleft (red arrow) is much shallower than in the original fin. The bifurcation points (red circles) are shifted distally.
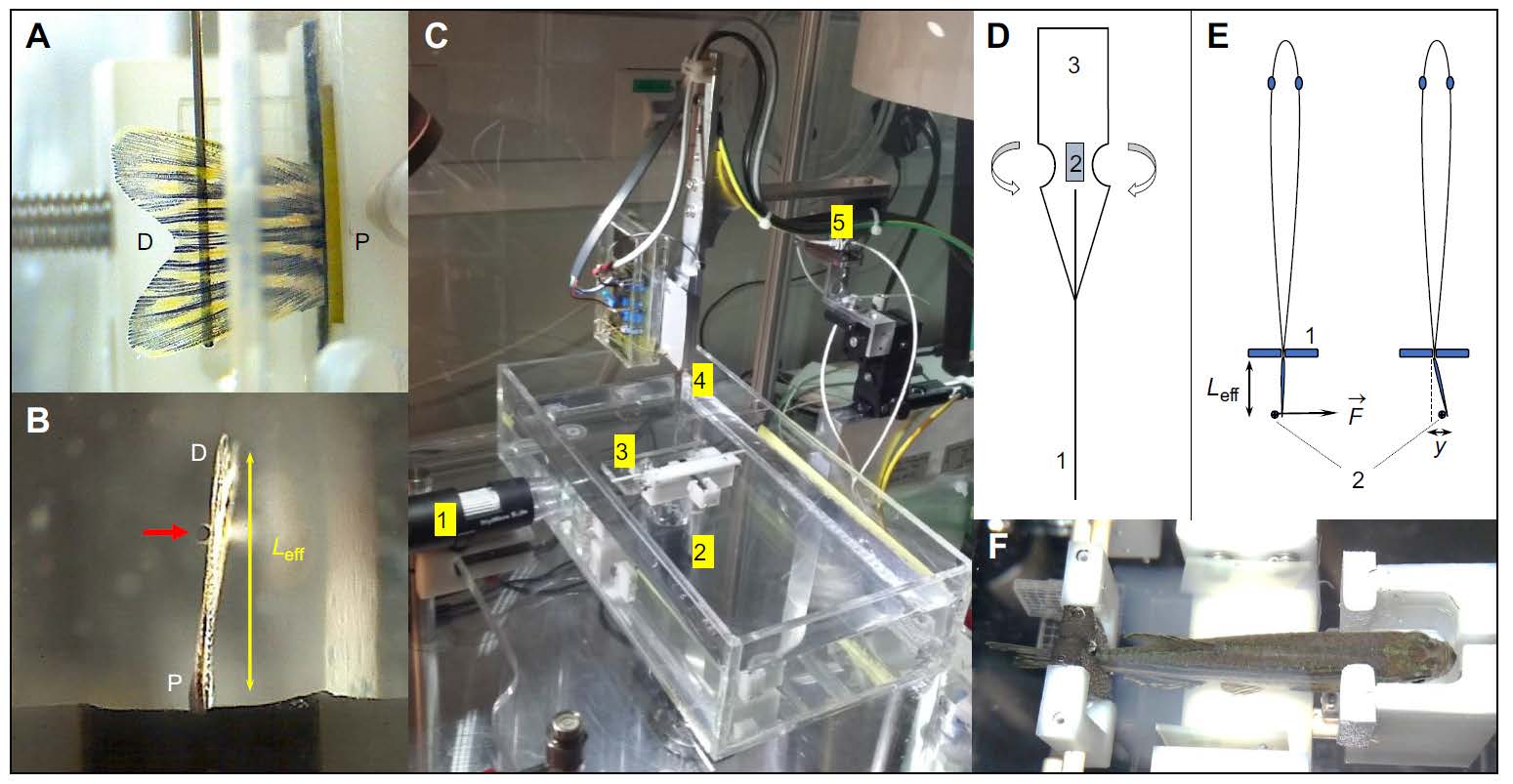
Setup overview describing the basic principle of measurement. (A) Side-view of a lined force attack from the indentation pin onto the fin surface. (B) Bottom-view showing the pin at a certain effective beam length from the fixation pad. (C) Overall impression of the measurement setup showing a water basin, two camera positions as in (A) and (B), a fish fixation device as in (F) and the sensor construct mounted on piezo-based positioners. (D) Design of the strain gage sensors mounted onto a spring sheet together with the indentation pin leading to the sensor base to increase the bending moment. (E) Measurement principle illustrating an indentation pin applying a force at a certain proximal-distal position (effective beam length, Leff) leading to a certain displacement y. (F) Fixation device hosting an anesthetized zebrafish.
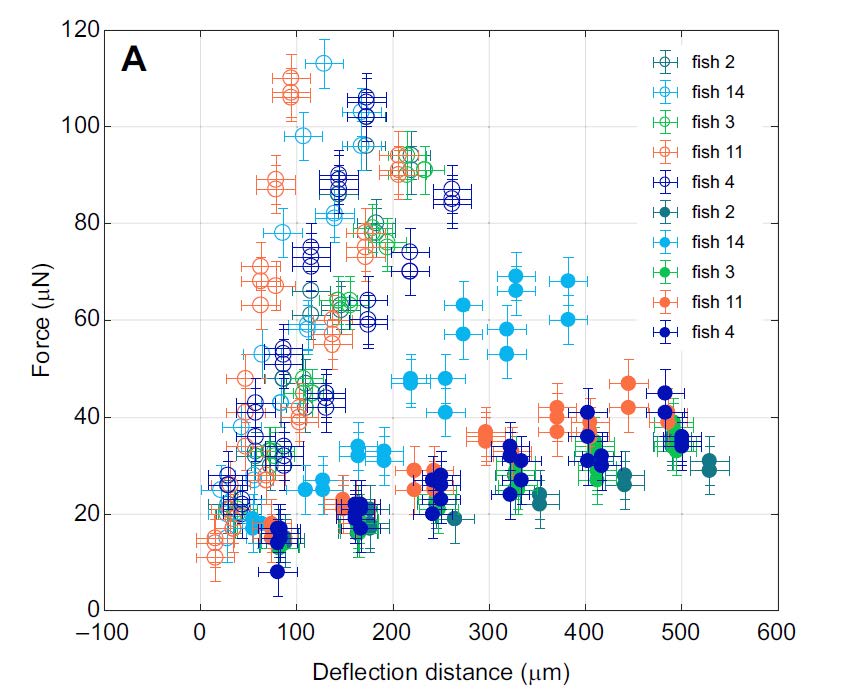
This allows a measure of the mechanical properties of the live zebra-fish fins in terms of its bending stiffness, which is determined from a force deflection curve similar to the one shown below. This bending stiffness will be mostly determined by the mechanical properties of the bony rays and hence will give a characterization of their effective elastic modulus.
When determining force deflection curves of fins, which are only partially bent in their area, leads to additional stretching of the soft interray tissue. The isolated response of this tissue is then obtained by surgically removing the tissue and bending the same part of the fin as before. As can be seen in the figure above, the force deflection curves including the stretching of the interray tissue show a higher spring constant by 0.4(1) N/m, thus determining the properties of the interray tissue.
These data are important inputs into coupled simulations of flows and elastic structures of the swimming fish in order to determine the forces acting on the fins during regeneration, which are carried out in collaboration with the Koumoutsakos lab at ETH Zurich.